by Sándor Demes, on
Presentation of the conference

The aim of this conference series is to highlight experimental and theoretical aspects of atomic and molecular interactions. At MOLEC 2022, the focus was on the following topics:
- Astrophysics and -chemistry
- Chirality
- Clusters and droplets
- Molecular collisions and reactions
- Structural dynamics
- Ultrafast electron dynamics
- Ultracold chemistry
- Ultrafast surface chemistry and collisions
The conference has been organized by Francesca Calegari, Jochen Küpper, Andrea Trabattoni, Vincent Wanie, Sebastian Trippel and Andrey Yachmenev.
The MOLEC 2022 welcomed 125 participants from 16 different countries.
47 talks and 52 posters have been presented during the week.
S. Demes presented his work in an oral presentation during this conference.
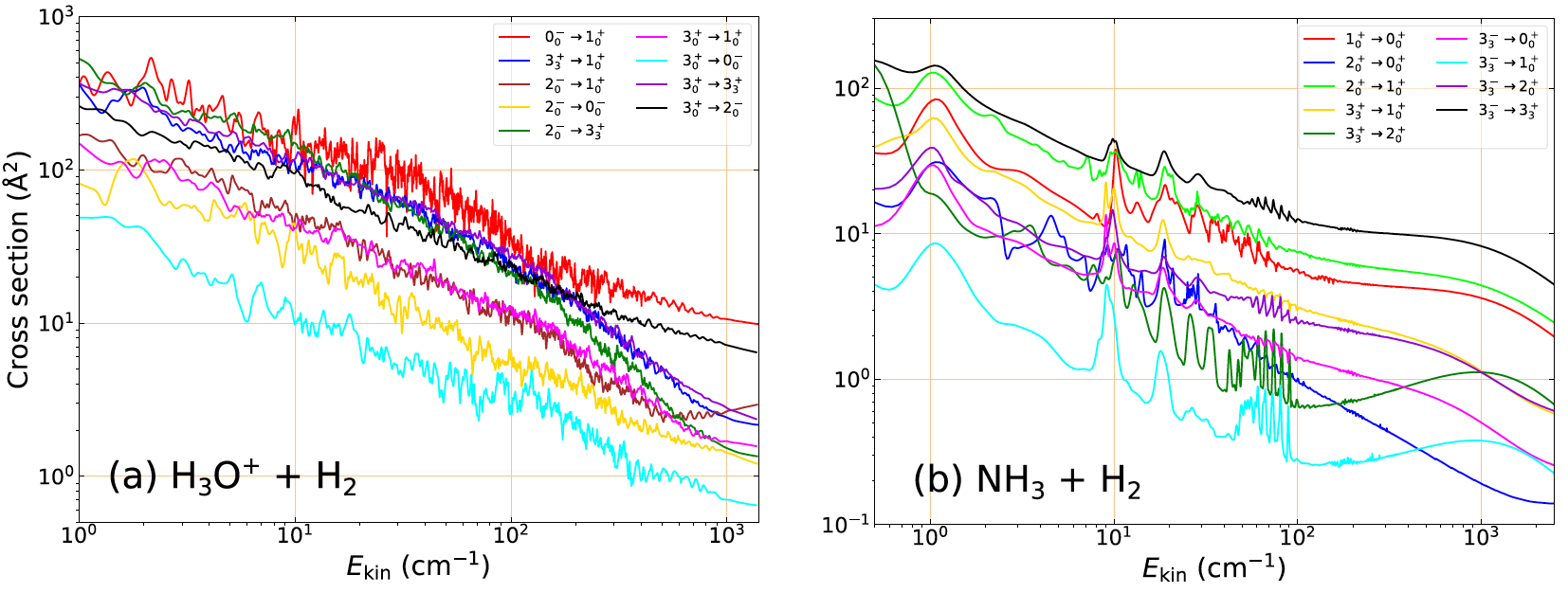
State-to-state cross sections and rate coefficients for the rotational excitation of polyatomic hydrides in astrophysical environments - S. Demes and F. Lique
Recent astronomical observations have been showed that interstellar molecular clouds exhibit a very rich and complex chemistry [1]. In order to understand in details the chemical composition in such environments, a non-LTE analysis of the emission spectra is necessary. This requires reliable determination of collisional rate coefficients for the transitions between rotational levels of the target molecular species.
Both the isoelectronic hydronium cation (H3O+) [2] and ammonia molecule (NH3) [3] have been detected in different regions of the interstellar medium (ISM). While H3O+ plays a crucial role in oxygen and water chemistry in the ISM, NH3 has been widely used as a probe of physical conditions in interstellar environments. Thus, studying their collisional excitation is of high importance for the proper interpretation of astrophysical observations.
The rotational excitation of H3O+ in collision with H2 molecule is studied for the first time [4]. State-to-state rotational de-excitation cross sections were computed using the close-coupling method, based on a highly accurate 5D potential energy surface [5]. The thermal rate coefficients were then derived up to 300 K kinetic temperatures.
We also present collisional data obtained for the excitation of NH3 by H2. We have noticeably extended the range of collision energies (up to 4500 cm1) and kinetic temperatures (up to 500 K), compared to previous studies by Bouhafs et al. [6] for this system. A comparative analysis of cross sections and rate
coefficients is provided for the H3O+ and NH3 target molecules. The calculated rate coefficients are used for modeling the collisional excitation of these species in interstellar molecular clouds.
[1] D. Flower, Molecular Collisions in the Interstellar Medium (Cambridge U.P., Cambridge, 2007).
[2] F.F.S van der Tak, S. Aalto, and R. Meijerink, A&A 477, L5 (2008).
[3] A.C. Cheung, D.M. Rank, C.H. Townes et al., PRL 21, 1701 (1968).
[4] S. Demes, F. Lique, F.F.S. van der Tak, A. Faure, C. Rist, P. Hily-Blant, MNRAS 509, 1252 (2022).
[5] S. Demes, F. Lique, A. Faure, C. Rist, J. Chem. Phys. 153, 094301 (2020).
[6] N. Bouhafs, C. Rist, F. Daniel, F. Dumouchel, F. Lique et al., MNRAS 470, 2204 (2017)